Why does a party balloon stick to the wall after you rub it on your head? Static electricity, of course! But why does it really happen? It occurred to me while researching electric machines from the 1740s (like Abbé Nollet’s machine featured in Ep. 1) that aside from vague recollections from Physics 101 of glass rods and rabbit fur, I didn’t have any kind of intuitive understanding of static electricity, specifically the contact or frictional variety, which has its own nifty word: triboelectricity.
The prefix ‘tribo-‘ comes from the Greek word τρίβος, which means ‘rubbing’ (according to the OED), and it was a word I’d never come across before. I had wanted to include a brief explanation of how it works in the Nollet piece, but it just didn’t fit, so instead it gets a whole post to itself!
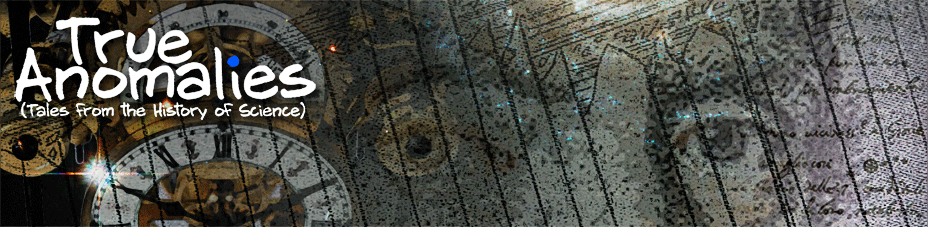
Nollet’s machine éléctrique is pretty straightforward. One person turns the large wheel, which causes a glass globe to rotate (very quickly, given the ratio of the wheel diameter to the diameter of the spindle holding the globe) at the other end. A second person, the woman in this image, holds a wool cloth against the globe as it spins, and a metal bar – suspended on threads to keep it insulated – is placed in contact with the glass (shown below). The woman and the glass globe are trading electrons as the glass whirls about – she’s gaining them and the globe is losing them. Why does it happen that way and not the other way around?
The short answer: the glass and the wool are made up of different kinds of atoms. (Well, yeah – no one’s surprised by that, right?) Each kind of atom has a unique number of protons (that’s what makes it a distinct element), and hence a unique number and configuration of electrons (neutral atoms at least). As a result, it’s a little bit easier for some atoms to accept extra electrons than others, and other atoms are better at giving up an electron or two – a property called charge affinity (although for atoms that are part of a molecule, electronegativity is maybe more appropriate).
That’s what’s going on with the wool and the glass. When they come into contact with each other, some points on that interface are so close together that the atoms actually form an adhesive bond, and when that happens they start to exchange charges with each other. When the contact is broken, one side can be left with extra electrons borrowed from the other, and the more times the contact is made and then broken, the more charge can build up. That’s why the electric machine is so effective – by spinning the globe quickly, you’re able to make and break contact a gazillion times with very little effort!
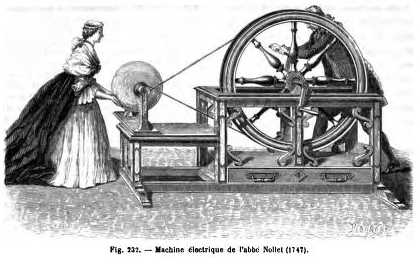
Once you have all this charge built up on the glass, you can feed it into the Leyden jar by connecting the metal bar (the “prime conductor”) to a wire that hangs down inside the jar. As long as you’re holding it (and as long as you’re grounded), the built-up charge inside the jar will cause an equal and opposite charge to build up on the outside. Ta-da! You now have a loaded Leyden jar, ready to deliver a punch to anyone who completes the circuit.
Some trivia:
1) Nollet and his contemporaries had a special tool for discharging the jar that allowed them to touch the outside of it to the wire hanging inside.
2) You can link many Leyden jars together to pack a bigger punch, and that’s where the modern usage of the word “battery” comes from! Ben Franklin applied the military term to the set of jars, which reminded him of a collection of guns.
3) The word root ‘electro-‘ comes from the Greek ήλεκτρον, which means ‘amber’ – a material that has been known since antiquity to build up charge if you rub it with a cloth like silk or leather.
Sources: Balloons & Static Electricity; Electric Machine; Charging a Leyden Jar; Leyden Jar Being Discharged; Battery of six Leyden jars; see also the sources listed here.